 |
|||||
|
|
|
Tryptamine Carriers FAQ | |
|
by Petrus Pennanen with help from Michael from Melbourne. Hypertexture and ©ontinuing illustration effort via http://deoxy.org
|
|
 |
| MAO Inhibitors and Tryptamines |
| Synthesis of DMT Derivatives |
| Psychedelic Toads |
| The Fungi |
| The Plants |
| References |
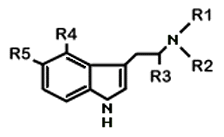
Orally and Parenterally Active Psychotropic Tryptamine Derivatives
Based on McKenna & Towers 1984
| Name of Compound | R1 | R2 | R3 | R4 | R5 | Dosage (mg) |
Route Oral/Par. |
|---|---|---|---|---|---|---|---|
| tryptamine | H | H | H | H | H | 100 *1 | par/oral? |
| DMT (dimethyltryptamine) | CH3 | CH3 | H | H | H | 60 | par |
| DET | C2H5 | C2H5 | H | H | H | 60 | par/oral |
| DPT | n-prop | n-prop | H | H | H | 60 | par/oral |
| DAT | C3H5 | C3H5 | H | H | H | 30 | par/oral |
| DIPT | i-prop | i-prop | H | H | H | 30 | oral |
| 5-MeO-DIPT | i-prop | i-prop | H | H | OCH3 | 12 | oral |
| 5-MeO-DMT | CH3 | CH3 | H | H | OCH3 | 6 | par |
| psilocin | CH3 | CH3 | H | OH | H | 12 *2 | oral |
| CZ-74 | C2H5 | C2H5 | H | OH | H | 15 *2 | oral |
| serotonin | H | H | H | H | OH | 100 *3 | oral |
| bufotenine | CH3 | CH3 | H | H | OH | 16 *4 | par |
| IT-290 | H | H | CH3 | H | H | 30 | oral |
| 4-hydroxy-alfa-methyl-tryptamine | H | H | CH3 | OH | H | 20 *3 | oral |
| MP-809 | H | H | CH3 | H | CH3 | 60 *5 | oral |
| 5-fluoro-alfa-methyl-tryptamine | H | H | CH3 | H | F | 25 *6 | oral |
| 5-methoxy-alfa-methyl-tryptamine | H | H | CH3 | H | OCH3 | 3 | oral |
| 4-hydroxy-diisopropyl-tryptamine | i-prop | i-prop | H | OH | H | 12 *6 | oral |
| 4-hydroxy-N-isopropyl, N-methyl-tryptamine | i-prop | CH3 | H | OH | H | 6 *6 | oral |
| N-t-butyl-tryptamine | H | t-butylH | H | H | ? | *7 | par? |
| 3-(2-(2,5-dimethylpyrrolyl) ethyl)-indole |
H | H | H | ? | ? | ? | ? |
| 5-alfa-DMT | CH3 | CH3 | CH3 | H | H | ? | ? |
- Autonomic symptoms; little central activity.
- The phosphate esters are psilocybin and CEY-19, respectively;
both are stoichiometrically equivalent to the 4-hydroxy isomers.
- Cardiovascular and autonomic symptoms; little central activity.
- A pressor amine rather than a hallucinogen in man.
- An antidepressant rather than a hallucinogen in man.
- Based on anonymous reports in the lay press.
No clinical studies have been published.
- No oral activity with doses up to 20 mg, may be parenterally active.
- Other potentially psychedelic tryptamines include:
- 6-fluoro-alfa-methyltryptamine, 7-methyltryptamine, 5-methyltryptamine
5-fluorotryptamine, 6-fluorotryptamine and 5- and 6-fluorotryptophans.
Search this FAQ for table of contents MAO Inhibitors and Tryptamines
Monoamine oxidase (MAO) is the primary inactivation pathway of most tryptamines. Because of this, inhibitors of the MAO enzyme (MAOIs) can be used to potentiate the effects of tryptamines and to make DMT and 5-MeO-DMT orally active.
MAO inhibitors fall into two classes: Irreversible and reversible MAOIs. In addition they can inhibit either or both of the two types of the MAO enzyme, MAO-A and MAO-B which are associated with serotonergic and dopaminergic neurons respectively. Irreversible MAOIs (e.g. the hydrazides iproniazid and phenelzine) bind permanently to the enzyme and cause MAO inhibition lasting 1-2 weeks after ingestion. They are used clinically to treat depression. Reversible MAOIs, such as moclobemide, which is used as an antidepressant, and the beta-carbolines harmine and harmaline, are effective for much shorter time, maybe up to 24 hours. Recreational drug users around the world are using mainly harmine and harmaline despite the lack of scientific studies on their effects on humans.
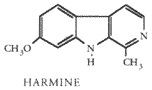
Natives of Amazon have traditionally combined Banisteriopsis caapi vine, which contains harmine, harmaline and related beta-carbolines, with DMT-containing plants to make an orally active brew called ayahuasca. Other plants containing harmine and/or harmaline can be substituted for B. caapi. The usual "North American ayahuasca" consists of Peganum harmala seeds and Desmanthus illinoensis roots, and in Australian "acaciahuasca" leaves of Acacia complanata are combined with material from DMT-containing acacias (the effectivity of this mixture hasn't been confirmed). MAOIs have also been used to potentiate the effects of mushrooms containing psilocybin. Terence McKenna has mentioned chocolate being a weak MAOI, which could be a reason for the popular habit of ingesting mushrooms with cocoa.
Peganum harmala (Syrian rue) seeds are the most concentrated natural source of harmine and harmaline—about 3% of their weight consists of these alkaloids. Banisteriopsis caapi has been found to contain from 0.18% to 1.36% beta-carbolines, with the concentration of harmine being from 0.057% to 0.635% (McKenna et al. 1984). According to anecdotal reports one gram of P. harmala seeds ingested inhibits MAO enough to make DMT orally active.
Harmine and harmaline are hallucinogenic on their own with doses starting from around 300 mg (Naranjo 1967), but often cause physical side-effects such as nausea and tremors in this dose range. They have little emotional or "psychedelic" effects, but produce strong visual hallucinations. Because of this the natives of Amazon often add larger amounts (75-100 cm of stem per dose) of B. caapi to ayahuasca brew than is needed for MAO inhibition (Luna 1984).
There are significant dangers in using MAO inhibitors. Most MAOIs potentiate the cardiovascular effects of tyramine and other monoamines found in foods. Ingestion of aged cheese, beer, wine, pickled herring, chicken liver, yeast, large amounts of coffee, citrus fruits, canned figs, broad beans, chocolate or cream while MAO is inhibited can cause a hypertensive crisis including a dangerous rise in blood pressure. Effects of amphetamines, general anaesthetics, sedatives, anti-histamines, alcohol, potent analgesics and anticholinergic and antidepressant agents are prolonged and intensified. Overdosage of MAOIs by themselves is also possible with effects including hyperreflexia and convulsions.
Search this FAQ for table of contents Synthesis of DMT Derivatives
Tryptamine derivatives and beta-Carbolines have been detected as endogenous metabolites in mammals, including humans. Methyl transferases that catalyze the synthesis of tryptamines, including DMT, 5-MeO-DMT and bufotenine, are found in human lung, brain, cerebrospinal fluid, liver and heart (McKenna & Towers 1984). In the pineal gland MAO is the primary inactivation pathway of serotonin, a neurotransmitter synthesized from the amino acid tryptophan. If MAO is blocked by harmine, harmaline or other MAO inhibitors serotonin can be converted by the methyltransferase enzymes HIOMT and INMT into psychedelic tryptamines (serotonin --(HIOMT)--> 5-MeO-trypt. --(2*INMT)--> 5-MeO-DMT).
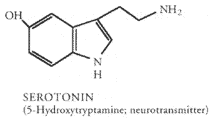
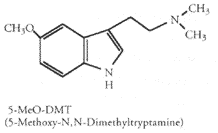
So, ingesting l-tryptophan to increase serotonin levels, a candy bar to increase the amount of tryptophan getting to your brain and natural plant material containing 25-50 mg harmine/harmaline to block MAO, all at the same time, might cause your pineal gland to synthesize substantial amounts of 5-MeO-DMT (Most 1986). This is extremely dangerous for persons with existing amine imbalance or schizophrenia. For normal, healthy people possible consequences are bad. DON'T TRY THIS.
A potent inhibitor of INMT, which is a necessary enzyme for the synthesis of DMT and 5-MeO-DMT, is found in particularly high concentrations in the pineal gland. A bypassing or inhibition of the synthesis of this inhibitor might be responsible for trances and other psychedelic states achieved "without drugs" (Strassman 1990). See Strassman's article for more info and speculation about the pineal gland.
Search this FAQ for table of contents Psychedelic Toads
Bufotenine and related 5-hydroxy-indolethylamines are common constituents of venoms of the genera Hyla, Leptodactylus, Rana and Bufo. Bufotenine is not psychedelic in reasonable doses (with larger doses there are dangerous physiological side effects), but the skin of one species, Bufo alvarius, contains 50-160 mg 5-MeO-DMT/g of skin (Daly & Witkop 1971). It's the only Bufo species known to contain a hallucinogenic tryptamine (McKenna & Towers 1984). Most (1984) gives instructions for collecting and drying the venom:
Fresh venom can easily be collected without harm to the toad. Use a flat glass plate or any other smooth, nonporous surface at least 12-inches square. Hold the toad in front of the plate, which is fixed in a vertical position. In this manner, the venom can be collected on the glass plate, free of dirt and liquid released when the toad is handled.When you are ready to begin, hold the toad firmly with one hand and, with the thumb and forefinger of your other hand, squeeze near the base of the gland until the venom squirts out of the pores and onto the glass plate. Use this method to systematically collect the venom from each of the toad's granular glands: those on the forearm, those on the tibia and femur of the hind leg, and, of course, the parotids on the neck. Each gland can be squeezed a second time for an additional yield of venom if you allow the toad a one-hour rest preiod. After this the glands are empty and require four to to six weeks for regeneration.
The venom is viscous and milky-white in color when first squeezed from the glands. It begins to dry within minutes and acquires the color and texture of rubber cement. Scrape the venom from the glass plate, dry it thoroughly, and store it in an airtight container until you are ready to smoke it.
Davis and Weil (1992) smoked the venom and described what happened:
In comparison to the pure compounds the toad venom appears longer lasting and, because one does not completely lose contact with reality, far more pleasant, even sensual. Shortly after inhalation I experienced warm flushing sensations, a sense of wonder and well-being, strong auditory hallucinations, which included an insect-cicada sound that ran across my mind and seemed to link my body to the earth. Though I was indoors, there was a sense of the feel of the earth, the dry desert soil passing through my fingers, the stars at midday, the scent of cactus and sage, the feel of dry leaves through hands. Strong visual hallucinations in orblike brilliance, diamond patterns that undulated across my visual field. The experience was in every sense pleasant, with no disturbing physical symptoms, no nausea, perhaps a slight sense of increased heart rate. Warm waves coursed up and down my body. The effects lasted only a few minutes but a pleasant afterglow continued for almost an hour. (Wade Davis, personal observation, January 12, 1991)Other animals contain DMT such as the gorgonian Paramuricea chamaeleon (Cimino & De Stefano, 1978).Profound alteration of consciousness within a few seconds of exhaling. I relax into a deep, peaceful interior awareness. There is nothing scary about the effects and no sense of toxicity. I try to describe my feelings but am unable to talk for the first five minutes and then only with some difficulty. This is a powerful psychoactive drug, one that I think would appeal to most people who like the effects of hallucinogens. For the next hour I feel slow and velvety, with a slight pressure in my head. No long-lasting effects to report. (Andrew T. Weil, personal observation, January 12, 1991).
Search this FAQ for table of contents
F U N G I |
|
|
|
|
|
|
|
|
|
|
|
|
|

|
| table of contents |
P L A N T S |
|
|
|
|
|

|
|
|
|
|
|

|
|
|
|
|
|
|
|
|

Amazonian Colombia natives roll small pellets of boiled resin in a evaporated filtrate of bark ashes of Gustavia Poeppigiana and ingest them to bring on a rapid intoxication (Smith 1977, Schultes 1977). |
|
|


|
|

|
|
|
|
|
|
References
Abu Zarga, M.H. 1986. Three new simple indole alkaloids from Limonia acidissima. Lloydia 49(5), 901-904.Akers, B.P. 1992. Peele's Lepiota: An identification and a clarification. Mycotaxon 43(0), 461-469.
Arora, D. 1986. Mushrooms Demystified: A Comprehensive Guide to the Fleshy Fungi. Ten Speed Press, Berkley.
Arthur, H.R., Loo, S.N. & Lamberton, J.A. 1967. Nb-methylated tryptamines and other constituents of Acacia confusa Merr. of Hong Kong. Aust. J Chem. 20, 811.
Banerjee, P.K. & Ghosal, S. 1969. Simple indole bases of Desmodium gangeticum. Aust. J Chem. 22, 275-277.
Barrau, J. 1958.
Nouvelles observations au sujet des plantes hallucinogenes d'usage autochtone en Nouvelle-Guinee. J Agric. Trop. Bot. Appl. 5, 377-378.Barrau, J. 1962.
Observations et travaux recents sur les vegetaux hallucinogenes de la Nouvelle-Guinee. J Agric. Trop. Bot. Appl. 9, 245-249.Benedict, R.G., Brady, L.R., Smith, A.H. & Tyler, V.E. 1962.
Occurrence of psilocybin and psilocin in certain Conocybe and Psilocybe species. Lloydia 25, 156-159.Benedict, R.G., Tyler, V.E. & Watling, R. 1967.
Blueing in Conocybe, Psilocybe and a Stropharia Species and the Dectection of Psilocybin. Lloydia 30(2), 150-157.Besl, H. 1993.
Galerina steglichii spec. nov., a hallucinogenic Galerina. Zeitschrift fuer Mykologie 59(2), 215-218.Bresinsky, A. & Besl, H. 1990.
A Colour Atlas of Poisonous Fungi. Wolfe Publishing Ltd, London.Bye, R.A. 1979.
Hallucinogenic plants of the Tarahumara. J. Ethnopharmacology 1, 23-48.Christiansen, A.L., Rasmussen, K.E. & Hoeiland, K. 1984.
Detection of psilocybin and psilocin in Norwegian species of Pluteus and Conocybe. Planta Med. 50, 341-343.Cimino, G. & De Stefano, S. 1978.
Chemistry of Mediterranean Gorgonians. Simple indole derivatives from Paramuricea chamaeleon. Comptes Rendus Biochem. Physiol. Ser. C. 61, 361-362.Crouch, I.J., Smith M.T., Van Staden J., Lewis, M.J. & Hoad, G.V. 1992.
Identification of auxins in a commercial seaweed concentrate. J. Plant Physiology 139(5), 590-594.Daly, J.W. & Witkop, B. 1971.
Chemistry and pharmacology of frog venoms. In Venomous animals and their venoms. Vol II. New York: Academic Press.Davis, W. & Weil, A.T. 1992.
Identity of a New World Psychoactive Toad. Ancient Mesoamerica 3 (1992) 5, 51-59.De Moraes, E.H.F., Alvarenga, Z.M.A., Ferreira, Z.M.G.S. & Alisue, G. 1990.
Quim. Nova 13, 308.Fitzgerald, J.S. & Sioumis, A.A. 1965.
Alkaloids of Australian Leguminosae V. Aust. J Chem. 18, 433.Fiussello, N. & Ceruti-Scarti, J. 1971/72.
Presenza di psilocibina edi 5-idrossi-indolderivati in Panaeolus retirugis. Atti Acc. Sci. Torino 106, 725-735.Gartz, J. 1991.
Influence of phosphate on fruiting and secondary metabolism of mycelia of Psilocybe cubensis, Psilocybe semilanceata and Gymnopilus purpuratus. Zeitschrift fuer Mykologie 57(1), 149-154.Ghosal, S., Chaudhuri, R.K., Dutta, S.K. & Bhattacharya, S.K. 1972.
Occurrence of curaromimetic indoles in the flowers of Arundo donax. Planta Med. 21, 22.Ghosal, S. & Mukherjee, B. 1966.
Indole-3-alkylamine Bases of Desmodium pulchellum. J. Org. Chem. 31, 2284.Ghosal, S., Singh, S. & Bhattacharya, S.K. 1971.
Alkaloids of Mucuna pruriens, Chemistry and Pharmacology. Planta Med. 19, 279Grina, J.A. et al. 1982.
Constituents of Zanthoxylum aborescens. Part 7. Old & new alkaloids from Zanthoxylum aborescens. J. Organic Chemistry 47, 2648-2651.Gurevich, L.S. 1993.
Indole derivatives in certain Panaeolus species from East Europe & Siberia. Mycological Research 97(2), 251-254.Gurevich, L.S. & Astapenko, V.V. 1992.
Chromatographic study of some indole metabolites in Panaeolus basidiomycetes. Mikologiya I Fitopathologiga 26(3), 189-194.Guzman, G. 1983.
The Genus Psilocybe. Beihefte Zur Nova Hedwingia 74, 1-439.Guzman, G., Bandala, V.M. & Allen, J.W. 1993.
A New Bluing Psilocybe from Thailand. Mycotaxon 26, 155-160.Guzman, G., Bandala, V.M. & King, C. 1991.
A New Species of Psilocybe of Section Zapotecorum from New Zealand. Mycological Research 95, 507-508.Guzman, G., Saldarriaga, Y., Pineda, F., Garcia, G. & Velazquez, L.F. 1994.
New Species of Psilocybe from Colombia and Discussion on the known species. Mycotaxon, 225.Haeselbarth, G., Michaelis, H. & Salnikow, J. 1985.
Nachweis von Psilocybin in Inocybe aeruginescens. Mykol. Mitt. bl. 28(1), 59-62.Hatfield, G.M., Valdes, L.J. & Smith, A.H. 1978.
The occurrence of psilocybin in Gymnopilus species. Lloydia 41, 140-144.Hein, R. & Wasson, R.G. 1958.
Les champignons hallucinogenes du Mexique. Museum National d'Histoire Naturelle, Paris.Holmstedt, B., Lindgren, J.E., et al. 1980.
Indole alkaloids in Amazonian Myristicaceae: Field and laboratory research. Bot. Mus. Leafl., Harvard Univ. 28, 215-234.Johns, S.R., Lamberton, J.A. & Sioumis, A.A. 1966.
Alkaloids of the Australian Leguminosae VI. Aust. J. Chem. 19, 893.Jossang, A., Jossang, C., Hadi, H.A., Sevenet, T. & Bodo, B. 1991.
Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Organic Chem. 56(23), 6527-6530.Kan Fan, C. et al. 1970.
Alcaloides de Vepris ampody (Rutacees). Phytochem. 9, 1283-1291.Kantor, R.E., Dudlettes, S.D. & Shulgin, A.T. 1980.
5-Methoxy-alfa-methyl-tryptamine (alfa,O-dimethylserotonin), a hallucinogenic homolog of serotonin. Biological Psychiatry Vol 15, 349-352.Koike, Y., Wada, K., Kusano, G., Nozoe, S., & Yokoyama, K. 1981.
Isolation of Psilocybin from Psilocybe argentipes and its Determination in Specimens of some Mushrooms. Lloydia 44(3), 362-365.Leboeuf, M. et al., 1977.
Alkaloids and triterpenes of Testulea gabonensis. Plant Medicine Phytotherapy 11, 230.Luna, L.E. 1984.
The Healing Practices of a Peruvian Shaman. J. Ethnopharmacology 11, 123-133.McKenna, D.J., Towers, G.H.N., & Abbott, F. (1984).
Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamines and Beta-carboline constituents of ayahuasca. J. Ethnopharmacology 10, 195-223.Mckenna, D.J. & Towers, G.H.N. 1984.
Biochemistry and Pharmacology of Tryptamines and beta-Carbolines: A Minireview. J. Psychoactive Drugs 16(4).Meckes-Lozoya, M., Lozoya, X., Marles, R.J., Soucy-Breau, C., Sen, A. & Arnason, J.T. 1990.
N,N-dimethyltryptamine alkaloid in Mimosa tenuiflora bark (tepescohuite). Arch. Invest. Med. Mex. 21(2), 175-7.Merlin, M.D. & Allen, J.W. 1993.
Species identification and chemical analysis of psychoactive fungi in the Hawaiian islands. Journal of Ethnopharmacology 40(1), 21-40.Miller Jr., O.K. 1972.
Mushrooms of North America. Dutton & Co., Springfield.Most, Albert. 1984.
Bufo Alvarius: the Psychedelic Toad of the Sonoran Desert. Venom Press Box 2863 Denton TX 76202.Most, Albert. 1986.
Eros and the Pineal: the layman's guide to cerebral solitaire. Venom Press, Denton, TX.Naranjo, C. 1969.
Psychotropic Properties of the Harmala Alkaloids. In: Efron (Ed.) The Ethnopharmacologic Search for Psychoactive Drugs.Ohenoja, E., Jokiranta, J., Makinen, T., Kaikkonen, A. & Airaksinen, M.M. 1987. The Occurrence of Psilocybin and Psilocin in Finnish Fungi. Lloydia 50(4), 741-744.
Pachter, I.J, Zacharias, D.E & Ribeir, O. 1959.
Indole Alkaloids of Acer saccharinum (the Silever Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 24, 1285-7.Phillips, R. 1981.
Mushrooms and other fungi of Great Britain and Europe. Pan Books, London.Poupat, C., Ahond, A. & Sevenet, T. 1976.
Alcaloides De Acaia simplicifolia. Phytochemistry 15, 2019-2120.Rivier, L. & Lindgren, J-E. 1972.
"Ayahuasca," the South American Hallucinogenic Drink: an Ethnobotanical and Chemical Investigation. Economic Botany 26, 101-129.Rivier, L. & Pilet, P.E. 1971.
Annee Biologique 10, 129.Robbers, J.E., Tyler, V.E. & Ola'h, G.M. 1969.
Additional evidence supporting the occurrence of psilocybin in Panaeolus foenisecii. Lloydia 32, 399-400.Rovelli, B. & Vaughan, G.N. 1967.
Alkaloids of Acacia I. Dimethyltryptamine in Acacia phlebophylla. Aust. J. Chem. 20, 1299-1300.Saupe, S.G. 1981.
Occurence of Psilocybin/Psilocin in Pluteus salicinus (Pluteaceae). Mycologia 73, 781-784.Schultes, R.E. 1976.
Indole Alkaloids in Plant Hallucinogens. J. of Psychedelic Drugs Vol 8(1), 7-25.Schultes, R.E. 1977.
The Botanical and Chemical Distribution of Hallucinogens. J. of Psychedelic Drugs Vol 9(3), 247-263.Schultes, R.E. 1979.
Hallucinogenic Plants: Their Earliest Botanical Descriptions. J. of Psychedelic Drugs Vol 11(1-2), 13-24.Schultes, R.E. & Hofmann, A. 1979.
Plants of the Gods. McGraw-Hill. Reprint available from Healing Arts Press, Rochester, VT.Schultes, R.E. & Hofmann, A. 1980.
The Botany and Chemistry of Hallucinogens. Springfield, Ill: Thomas.Shulgin, A.T. 1976.
Psychotomimetic agents. In: Gordon, M. (Ed.) Psychopharmacological Agents, Vol IV. New York: Academic Press.Shulgin, A.T. 1982.
Chemistry of Psychotomimetics. In: Hoffmeister, F. & Stille, G. (Eds.) Handbook of Experimental Pharmacology, Vol 55: Alcohol and Psychotomimetics, Psychotropic Effects of Central-Acting Drugs. New York: Springer-Verlag.Skaltsounis, A.L., Tillequin, F. & Koch, M. 1983.
Plantes des Nouvelle- Caledonie LXXXIII: Alcaloides des tiges feuillees de Melicope leptococca. Lloydia 46(5), 732.Smith, T.A. 1977.
Review: Tryptamine and Related Compounds in Plants. Phytochemistry 16 171-175.Stein, S.I. 1959.
Clinical Observations on the Effects of Panaeolus venenosus Versus Psilocybe caerulescens Mushrooms. Mycologica 51, 49.Stijve, T., Klan, J. & Kugper, W. 1985.
Occurrence of psilocybin and baeocystin in the genus Inocybe. Persoonia 12, 469-472.Strassman, R.J. 1990.
The Pineal Gland: Current Evidence For Its Role In Consciousness. In: Lyttle, T. (Ed.) Psychedelic Monographs and Essays Vol 5.Thompson, A.C., Nicollier, G.F. & Pope, D.F 1987.
Indolealkylamines of Desmanthus illinoensis and Their Growth Inhibition Activity. J. Agric. Food Chem. 35, 361-365.Wahba, S.K. & Elkheir, Y.M. 1975.
Dimethyltyrptamine from the leaves of certain Acacia species of northern Sudan. Lloydia 38, 176-177.Wassel, G.M. et al. 1985.
Alkaloids from the rhizomes of Phragmites australis Cav. Scientia Pharmaceutica 53, 169-170.Petrus.Pennanen@helsinki.fi * Everything is perfect forever
Michael from Melbourne * Ditto
 D E O X Y . O R G | |
| END OF FAQ | |